Mesoporous Silica
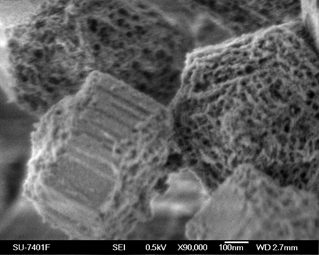
The aim of this project is to devellop a new class of engineered mesoporous silica particles (MPS) based materials to prevent and treat metabolic disease and disorders, including type 2 diabetes. The industrail partner involved in this project is Sigrid Therapeutics (Sigrid), which is a clinical-stage technology startup pioneering the development of a product candidate, SiPore15, an orally-administered medical device based on the Company’s proprietary platform technology, SiPore. Designed to act locally in the gut, SiPore15, consists of precisely engineered micron-sized silica particles with tailored porosity. Clinical data confirms SiPore15’s beneficial effects on a range of metabolic parameters and its excellent safety profile. Upon its approval, SiPore15 will be the first medical device available for reduction of blood sugar levels in people at risk of developing type 2 diabetes.
For more details please contact Niklas Hedin ( [email protected] ) and Sana Alajmovic ([email protected]), Sigrid Therapeutics AB (https://www.sigridthx.com/)